Is Hydroxyapatite Safe? The Muddy Waters of Nano and Microparticles
 Hydroxyapatite is having a moment in a big way. Touted as a natural replacement for fluoride, the mineral has been found to help reduce tooth sensitivity and fill in weak tooth enamel. But is hydroxyapatite safe? No one seems to be talking about the risks and long-term effects of this ingredient. So that's what we're going to do today.
Hydroxyapatite is having a moment in a big way. Touted as a natural replacement for fluoride, the mineral has been found to help reduce tooth sensitivity and fill in weak tooth enamel. But is hydroxyapatite safe? No one seems to be talking about the risks and long-term effects of this ingredient. So that's what we're going to do today.
What is Hydroxyapatite?
Hydroxyapatite is a naturally occurring calcium and phosphorous compound of which our teeth and bones are comprised. In the 1970s, NASA developed nano hydroxyapatite to help astronauts maintain healthy teeth and bones while they were in space. Scientists found that by creating hydroxyapatite nanoparticles, they could strengthen the body's natural hydroxyapatite structures. The ultra fine calcium/phosphorous crystals were able to insert and imbed themselves into to the surface of teeth and bone due to their extremely small size.
In the 1980s a Japanese company called Sangi Co. obtained the patent from NASA. In the 80's and 90s, it was used on a fairly small scale in Japan in toothpaste. In the early 2000s, the company figured out how to make the nano particles considerably smaller, and started marketing the product internationally as a toothpaste ingredient. A few companies in Europe started making hydroxyapatite toothpaste. In 2016 the EU started talking about banning the ingredient and in 2017 it hit the market in the US where it's completely unregulated instead.
Proponents of nano hydroxyapatite (N-HAP) state it’s a safe replacement for fluoride in toothpaste, however it works much in the same way. With fluoride, fluorapatite is created on the surface of the enamel. With n-HAP, nano sized particles are able to be absorbed by a layer of protective proteins on the enamel called the pellicle. These particles are so small that they can be caught and held by these proteins and then presumably release calcium ions that are incorporated into the tooth.
It's potentially great for teeth, but what about the rest of the body? Is hydroxyapatite safe?
While it may sound like a healthier alternative because hydroxyapatite exists in the body naturally, we have to remember we're dealing with nanoparticles.
What Animal Studies Have Found

In 2016, the European Commission Scientific Committee on Consumer Safety wanted to find out is hydroxyapatite safe. They compiled a number of studies. Their findings were eye-opening. All are excerpts below are from their document, found here.
Effects on Lungs
When considering is hydroxyapatite safe, we have to look at animal studies. Lab rats were fed high amounts of N-HAP with some disturbing findings:
Death of the animals occurred from 10,000 mg/kg bw in both sexes as a result of cardiac and respiratory arrest. Important findings on animals surviving 14 d: lung vessels were moderately congested, “alveoli filled with air”; signs of “fatty degeneration of the liver”.
While this was a very high dose, other studies with lower doses found NAHA affected lungs in similar way:
Histopathological evaluation revealed “pseudotubercles in lung, performing intracavity embolism, inflammatory cell infiltrate and hyperplasy of stroma cell.”
and:
In lungs, seven cases of bronchial associated lymphoid tissue proliferation were observed.
Even though the nanoparticles were either injected or ingested, the nanoparticles ended up in the animals' lungs and created an inflammatory response there.
p>In addition to lung inflammation, NAHA was found to create free radical damage in the liver.
[t]he liver tissues from the rats exposed to nanohydroxyapatite showed inflammatory cell infiltration at the portal areas of the liver. Haematological analysis demonstrated increased white blood cells, elevated levels of the inflammatory cytokine TNF-α, and increased alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bile acid (TBA), cholesterol, uric acid, lactate dehydrogenase (LDH) and low density lipoprotein (LDL) compared to controls. In the livers, increased levels of H2O2 and MDA (malondialdehyde) and decreased levels of glutathione (p<0.05) were observed.
Other markers of inflammation were found:
[T]here was a statistically significant increase in red blood cells, haemoglobin and haematocrit and a statistically significant decrease in platelet counts in treated animals. With respect to biochemical parameters, there were slight decreases in serum glutamic oxaloacetic transaminase, glucose and urea and statistically significant increases in serum glutamic pyruvate transaminase and alkaline phosphatase in treated animals compared to controls,
And that hydroxyapatite killed liver and kidney cells:
Histopathology revealed apoptotic cells in the kidneys and livers of animals treated with nano-hydroxyapatite.
Another study found free radical damage in the liver when exposed to NAHA:
Investigation of liver homogenates revealed changes in lipid peroxidation and reduced glutathione when compared to controls; however, there was no dose-dependency. All antioxidant enzymes investigated were lower compared to control with a dose-dependency for glutathione reductase. Further, superoxide dismutase was decreased in homogenates from treated animals.
Effects on Kidneys
Hydroxyapatite was also found to damage kidney tissue in animal studies:
1# nanomaterials (i.e. the smaller ones) resulted in a “vacuolar degeneration of nephric tubule epithelium in the kidney."
it can be deduced that systemically available nano-hydroxyapatite particles might be distributed into lungs and kidneys as histopathological changes were observed in these two organs.
Compared to untreated controls, dose-dependent increase of products of lipid peroxidation in liver, kidney and lung; significantly increased nitrate and nitrite levels in kidneys, livers and lungs; significantly reduced membrane fluidity in liver, kidney and lung; increase in ATPase activity in liver, kidney and lung (statistically significant after 20 mg in liver and after 10 and 20 mg in kidneys); statistically significantly increased glutathione peroxidase activity in erythrocytes from rats treated with 20 mg nano-HYAP.
Effects on Blood and Platelets
One study found N-HAP was able to negatively affect red blood cells and platelets because the nanoparticles stick to the cell membranes:
"Studies further demonstrate the induction of oxidative stress and inflammatory changes in cells after treatment with nano-hydroxyapatite. Thus, if systemically available, nano-hydroxyapatite might affect cells when it adheres to the plasma membrane from outside (e.g. of blood cells) or when it reaches the intracellular compartment"
"Studies using red blood cells have demonstrated that external adherence of nanohydroxyapatite to erythrocytes (which might be of relevance to the nano-hydroxyapatite material available in blood) might also have an impact on these cells (e.g. on sedimentation, aggregation)."
"there was a statistically significant increase in red blood cells, haemoglobin and haematocrit and a statistically significant decrease in platelet counts in treated animals."
Damage to DNA
One study found N-HAP damaged human DNA in vitro studies:
Turkez et al. (2014) investigated sister chromatid exchanges, micronucleus formation, chromosome aberration and 8-oxo-2-deoxyguanosine formation by needle-shaped nanohydroxyapatite with average particle diameters of 10 – 50 nm in cultured peripheral blood lymphocytes from 6 human donors. In comparison to untreated cultures, dose-dependent increases - in part of statistical significance - of sister chromatid exchanges, micronuclei, chromosome aberration rates and 8-oxo-2-deoxyguanosine levels were observed in treated cultures.
Size & Form Matter
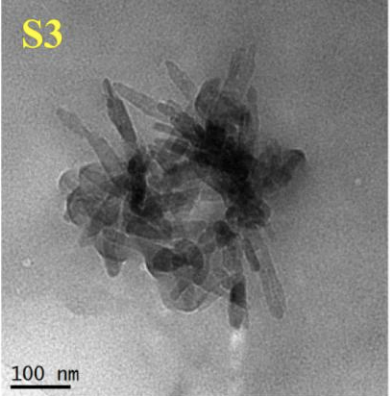
When trying to decide is hydroxyapatite safe, compiling the available data, it became clear that the size and shape of the particle was important. Smaller particles were more harmful as were needle-shaped particles. The SCCS concluded that there wasn't enough data to safely recommend the use of hydroxyapatite in general, and concluded that needle-shaped N-HAP should not be used at all in cosmetics.
"The available information indicates that nano-hydroxyapatite in needle-shaped form is of concern in relation to potential toxicity. Therefore, needle-shaped nano-hydroxyapatite should not be used in cosmetic products. It is of note that Material 2 of the submission also includes nanofibres of needle-like structure".
The problem for consumers, however, is that companies using N-HAP don't differentiate or disclose particle size or shape, and it's not always apparent if nanoparticles are being used.
Microcrystalline vs Nanoparticles: Muddy Waters
Most toothpaste companies using hydroxyapatite fully disclose and market that they're using nanoparticles. Others, it's not so clear.
One German company claims that their toothpaste doesn't contain nanoparticles and has published a couple studies showing their product remineralized teeth. Looking through their study, there was a mention of crystallite size. The crystallite size on average was 80 nanometers long by 30 nanometers wide, which falls into the size category of a nanoparticle. But a crystallite is not a particle. A crystal particle is made up of a clump of crystallites. So, while the components of the particle are nano-sized, the overall particle measurement is large enough to not be considered a nanoparticle. The fact that these clumps have nano-sized crystallites likely explains how a “micro” sized hydroxyapatite could remineralize teeth, as we basically have nano-sized spikes sticking out that are small enough to be utilized by the pellicle.
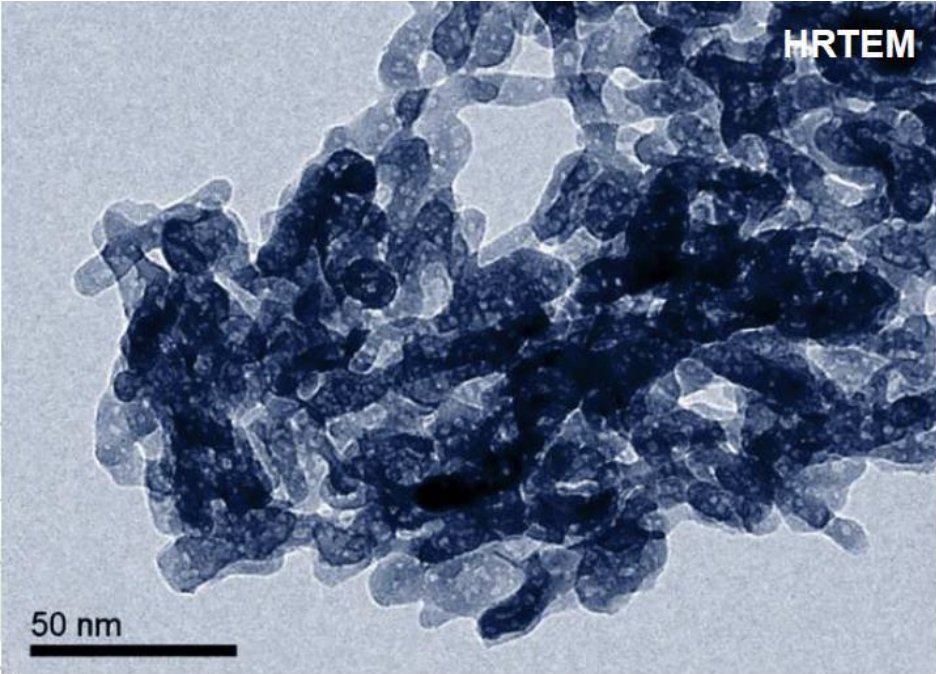
There is no data available on the American company that uses "micro" hydroxyapatite, and if their particles are similar. But, it stands to reason that if the formula is effective at remineralizing teeth, there’s something going on nano-scale, as larger particles wouldn’t be able to penetrate the pellicle of the enamel and be held to release calcium ions. Either that, or it's not terribly effective at rebuilding enamel.
The Story Gets more Disturbing

In my research I ran on to this study that looked at the presence of hydroxyapatite nanoparticles in baby formula. Researchers had caught wind of the SCCS’s recommendation that needle-shaped particles shouldn’t be used in cosmetics due to their toxic effects on the liver, lungs, kidneys and blood. What about our food supply, they asked? Is N-HAP finding its way into our foods for our most susceptible population: infants?
Noticing that many brands of infant formulas contained hydroxyapatite as a form of calcium and phosphorous supplementation, researchers with the Center for Food Safety set out to find if these formulas contained nanoparticles, specifically in the most harmful needle-shaped form. According to their findings, all of the samples contained nanoparticles of some sort. Three contained needle-shaped N-HAP, one contained spherical nano silicon dioxide, one contained unknown spherical nano calcium and phosphorous particles, and the last contained nano titanium dioxide and calcium.
In their research they also found that needle-shaped nano particles can clump together and, under a microscope, look like larger clumps of particles
"[The] samples contained needle-like shaped particles 10-30 nm in width and 100-300 nm in length, creating impressions of dendritic networks"
So upon first glance, these particles look like “micro” HA when in fact they’re N-HAP clumped together.
The baby formula study brings up a lot of questions. Do the formula companies intentionally purchase N-HAP because it’s easy to work with and dissolves easily? Or were they sold microcrystalline HA that ended up containing nanoparticles?
Researchers hypothesized that N-HAP could easily dissolve in the digestive system under the influence of stomach acid, although this theory has not been confirmed. And the previously mentioned animal studies may indicate otherwise. In light of those studies they theorized that:
"If there is a high concentration of phosphate and calcium ions in the small intestine under alkaline conditions, you can get precipitation of HA. This could impact the effects of HA on the gut microbiome because they would be exposed to non-dissolved (i.e., near pristine) forms of the ENP. [ENP standing for Engineered Nano Particle]"
The effects of N-HAP particles on the human gut are completely unknown.
There is no labeling requirement when it comes to differentiating types of hydroxyapatite. When it’s listed on a label it can be a large particle, microcrystalline, or nano. Companies aren’t required to disclose.
Legality Called into Question
Is hydroxyapatite safe? The it has not been fully tested for safety and efficacy. According to current data, remineralizing claims made by toothpaste companies may not be compliant with FDA regulations. "Remineralizing" claims may be drug claims and no hydroxyapatite product has been FDA-approved as a drug.

Questions Remain
Is hydroxyapatite safe? While companies selling n-HAP products claim the material is biocompatible, they're not considering how these nanoparticles affect certain cells, create free radical damage, and accumulate in organs. There is still much we don’t know about hydroxyapatite as it’s such a new technology. It has not been studied for carcinogenic potential, and limited data exists for its reproductive toxicity.
In my opinion, further study should be done to answer the following questions:
- What are the effects of N-HAP on the gut?
- Can N-HAP be absorbed sublingually?
- Can N-HAP pass the blood-brain barrier like other nanoparticles?
- If N-HAP is able to calcify blood cells, does it have the potential to calcify arteries? (UPDATE: Yes, it actually does: https://pubmed.ncbi.nlm.nih.gov/31831831/)
So much more needs to be known about its safety and nanoparticles should be disclosed and labeled.
Sources:
https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-019-0454-6
Recent Posts
-
Vegetable Emulsifying Wax NF
What is Vegetable Emulsifying Wax NF?Vegetable Emulsifying Wax NF is a blend of chemicals, namely ce …16th Aug 2024 -
Tocopherol vs. Tocopheryl Acetate: What's the Difference?
Let's look at the differences between tocopherol vs tocopheryl acetateThere are two forms of vitamin …16th Jul 2024 -
Are Essential Oils Toxic?
The following comment was made in a Facebook group for which I'm an admin. It was a lot to unpack in …16th Jul 2024
